
Get a Quote
Get a Quote and Find Services to Fit Your Needs 50000+ Satisfied Clients
5000+ Licenses & Registration
15 Branches across India
75 Years + Combined experience
Satisfied Clients
Services
Years Combined Experience
Get Started!

























Process of Drug License in India
Whether you are planning to enter the Pharma Industry or launch a Healthcare Entity, obtaining a Drug License from the respective Authority is something you can't afford to miss. According to the Drugs and Cosmetics Act, a drug license is usually allotted by the State of Central Drug Standard Control Organization.
The Central Drugs Standard Control Organization (CDSCO) administers the Drugs and Cosmetics Act to control substandard drugs and regulate the import, manufacturing, distribution, and sale of drugs and cosmetics through licensing. The D&C Act's Section 26B, which gives the CDSCO the Authority to control, regulate, or prohibit the manufacturing of medicine in the public interest, has recently been the subject of several notices.
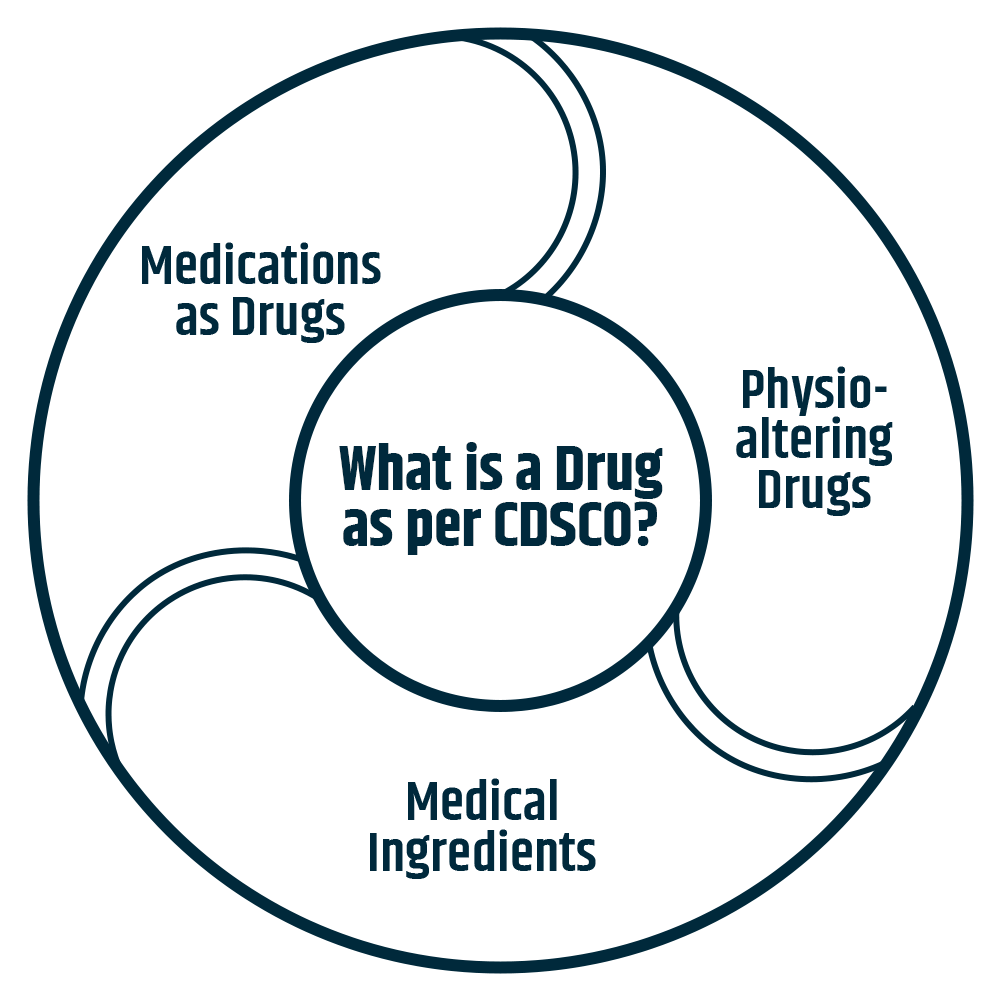
All medications intended for internal or exterior usage in humans or animals, as well as all compounds used in the treatment, mitigation, or prevention of any disease or ailment in humans or animals, including preparations to ward off insects like mosquitoes.
Drugs that the Central Government occasionally specifies by notification in the Official Gazette. Such substances (other than food) are intended to affect the structure or function of the human body or to be used to destroy 10 [vermin] or insects that cause disease in human beings or animals.
Drugs, including chemicals and empty gelatin capsules, are intended to be used as medicinal ingredients.
The Central Government may, from time to time, following consultation with the Board, specify such devices intended for internal or external use in the diagnosis, treatment, mitigation, or prevention of sickness or disorder in humans or animals.
Below are different types of drug licenses for the Medical Stores being issued by the concerned Authority.
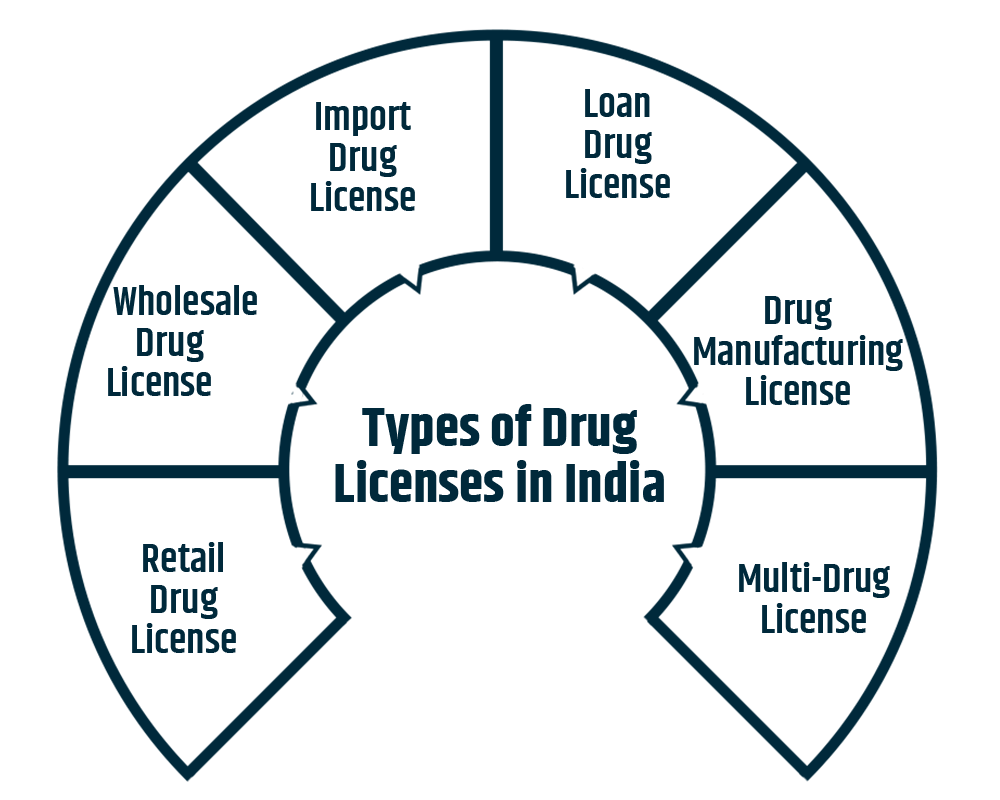
Retailers selling medications, independent pharmacists, etc., can apply for this License with the State Pharmacy Council. This usually refers to a drug license for a medical shop or a medical shop license.
This drug license is given by the central drugs standard control authority to wholesalers involved in the pharmaceutical business. The person applying for a wholesale drug license online should meet the following eligibility criteria:
As its name indicates, this License is sought by dealers who want to import raw materials for manufacturing drugs or import ready-to-sell drugs in India.
The drug manufacturer is willing to use his brand name to manufacture on a property for which a license has already been granted but does not own his land.
Every manufacturer of allopathic, cosmetic, Ayurveda, or any other pharmaceuticals or remedies as defined by the Drugs and Cosmetics Act of 1940. This is the state permit granted by the particular state administration where the property is located.
This drug license is meant for businesses operating in multiple states and units.
In this article, we are going to answer the question of how to get a drug license in India. We will also detail the specific requirements for wholesale and retail Drug Licenses in India.
One of India's two most prominent Pharma Licenses is Retail License. But what are Retailers? Retailers are generic store owners with delible expertise in selling medications for generic purposes. Every independent pharmacist can open a Retail Store by acquiring a Retail Pharma License. You can apply for a Retail License with the State Pharmacy Council.
But, such is not the case with acquiring a Wholesale Pharma License in India. CDSCO, or the Central Drugs Standard Control Authority, issues the Wholesale Pharma License. The wholesalers involved in the pharmaceutical business envisioning to open a chain of pharmaceutical stores across a geographical area opt for the said License. But, the applicant must keep a few things in mind before applying for a Wholesale Drug License online. First, the applicant must hold a diploma or degree in pharmacy and a minimum of a year's experience in drug sales and distribution.
This article specifically focuses on the wholesale and Retailer License registration procedures. But before applying for the requisite License, let us learn a bit about the required eligibility.
The requisite criteria for obtaining a Wholesale or Retail License for Medical Stores in India are detailed in the following points:
What qualifications are for getting a wholesale or retail license in India? Some essential qualifications are required from Wholesale or Retail License holders in India.
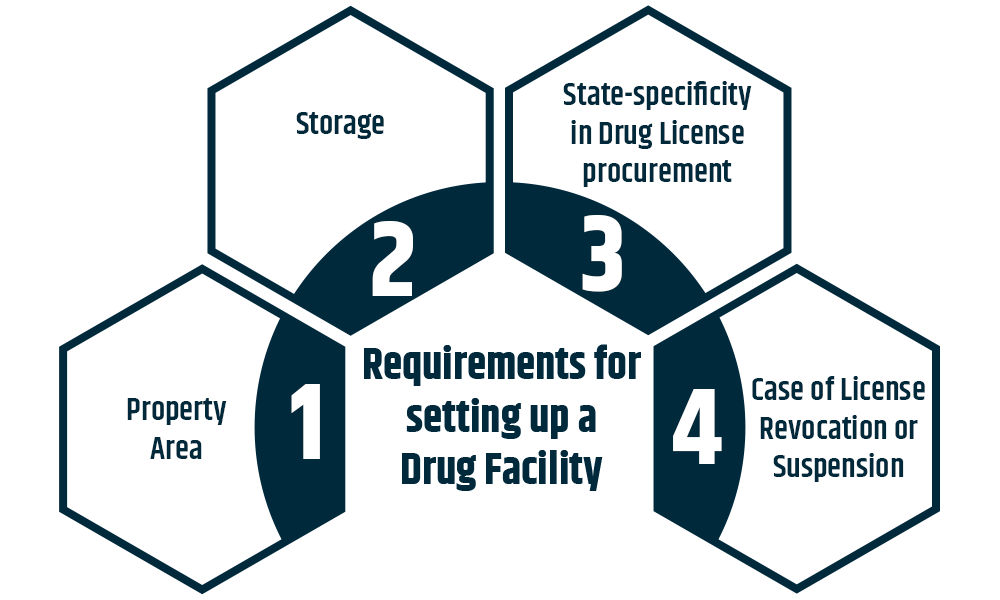
Setting up a pharmacy or medical store requires a minimum of 10 square meters. However, if they are running both a wholesale and a retail operation, a minimum of 15 square meters is needed.
Implementing cold storage, freezers, or similar facilities should provide proper storage for medications and vaccines, as they need to be kept in cool areas.
Depending on their line of work, companies that deal in medications or medicines may need a variety of Drug Licenses. A single drug dealer must obtain Drug Licenses with several units in more than one state since they are region-specific. Every location where drugs are kept and sold must apply for a drug license, except migrant vendors.
Holders of drug licenses must ensure that the product conforms to all rules and regulations. The License granted defying these terms and conditions may be revoked or suspended. In addition, authorities must be notified of any business-related changes or modifications. These are some qualifications required for online Wholesale or Retail Drug License registration.
Documents Required for Online Wholesale Drug License Registration in India
The documents required to apply for a Wholesale Drug License in India are mentioned below. Requirement of documents is different for applicants as per the nature of the service they require. Below is the list of complete documents:
Documents Required for Fresh Application:
*In case of a village, Khasra Khatauni documents will be considered.
Documents Required for Application of Additional Wholesale License
Documents required for Application of renewal of Retail/Wholesale license
Documents required for Change in Registered Pharmacist
To apply online for a wholesale or retail license, the applicant must follow the given basic procedure to obtain the required Food and Drug License in India:
Drug License Fee structure:
The licensing fee depends on the nature of the sought permit. Also, as always in the legal procedure, additional hidden charges, along with the requisite wholesale or retail drug license fee, must be paid for obtaining various lab tests or analyses as per the Central or State Drugs Laboratory's norms. In certain cases, the Pharmacopoeial Laboratory for Indian Medicine mandates testing procedures for the applicant to obtain the requisite drug license.
The drug license fee depends on several factors, such as the following:
Therefore, the aforementioned factors are considered before deducing the amount for paying the drug license fee to the Authority. If you wish to know the drug license fee in Delhi for wholesale or retail shop owners, then connect with the CDSCO experts at Registrationwala.
At Registrationwala, we have a team of skilled and experienced professionals who first understand your specific license requirements for both wholesaler and Retailer, the nature of the business, and other specifications, and then provide full-fledged support in license procurement. In addition, we ensure that your drug license registration is done proficiently so that it gets approved quickly without any hassle. So, apply for a wholesale or retail license online with Registrationwala.
When you apply for an online wholesale or Retail license, you get the following:
So, connect with our experts right away, and get the best advice on wholesale or retail license registration.
Q. How can one apply for retail drug license renewal online?
A. The process of renewing wholesale drug licenses is completely online. Follow the below mentioned steps to renew the certificate from the department.
Q. Who Can Apply for a Wholesale Drug License?
A. A person who fulfills the following qualification requirements can apply for a wholesale drug license:
Q. What are cost of wholesale drug license?
A. The cost of drug license in online payment is Rs. 6000/- + for fees of License and Inspection fees of Rs. 1500/-. This cost is for getting a Drug license in Delhi.
Q. What are the documents required for wholesale drug license registration in India?
A. Same as the documents required for a Retail Drug license in India, the required documentation is as follows:
Q. What area is required for attaining the wholesale drug license in India?
A. As one of the prominent Wholesale and Retail Drug License requirements, one is area specifications for office space. For example, every pharmacy/medical store requires 10 square meters of office space, but for running wholesale and retail operations, at least 15 square meters is required.
Q. How can one apply for retail drug license renewal online?
A. After paying for the Drug License Fee as applicable in Delhi, the drug distributor license holder worries about premature license expiry. To secure yourself from such setbacks, you can avail of Drug License renewal services at Registrationwala. Our Experts will brief you on the entire procedure.
Q. What is the procedure for online drug license verification in India?
A. The procedure for drug license verification is quite simple. The verification can be done online on the State's Drug Authority portal. Just enter the Drug License number to verify its details on the website. You can also download the wholesale sale drug license verification procedural manual from the respective portal.
Q. Is the requirement for Wholesale Drug License registration documents the same as retail drug license registration?
A. Yes, irrespective of the license category, the documentation requirements for all types of drug licenses are the same.
Q. Which Drugs are Banned in India for Wholesale Drug License?
A. There are multiple drugs which are banned from wholesale drug licenses. 10 drugs are prohibited for import some of them are Nialamide, Practolol, Amidopyrine, Rimonabant, etc. Check the complete banned list of drugs here.

★ ★ ★ ★ ★
I very much appreciate the fact that you guys possess tremendous knowhow of private limited company incorporation. You have exhibited professional and respectful manner towards my query and I would seriously recommend you guys to all the folks looking for outstanding business services.

★ ★ ★ ★ ★
Thanks to their support, I got my trademark successfully. I highly recommend their services for anyone needing help with their intellectual property. The person assigned to me was very cooperative and helpful.

★ ★ ★ ★ ★
Thanks to their support ragistrationwala team, I got my IP-1 license successfully and special thanks to Miss.Kanishka for your great and timing support !!!!!! I have archived my goal one step forward... Thanks for the entire team....

★ ★ ★ ★ ★
Really helped a lot in getting my both VNO licenses. Great experience working with the team and very humble team, thanks for providing the vno license on time.

★ ★ ★ ★ ★
I had a good time working with Registrationwala. Good team. I would recommend their services to others.

★ ★ ★ ★ ★
It was extremely great service of Registrationwala consulting firm, and this firm is providing the best services and worry about the client's required services along the client's satisfaction.

★ ★ ★ ★ ★
Superb Experince! Within no time the trademark registration was on.Highly professional team. I am very much Impressed with the prompt response and efficiency.Thank you.

★ ★ ★ ★ ★
We had taken ISP license from registration wala and the supporting person is very helpful to taken that license his communication and his work is satisfactory and thanks for those services

★ ★ ★ ★ ★
I sincerely appreciate your prompt support in helping me get the access license so quickly. Your professionalism and efficiency are truly commendable. Thank you for going above and beyond to assist me. Keep up the great work!